CAR-T cell therapy: Creating an investible patent strategy in a crowded market
CAR-T cell therapy, a transformative force in cancer treatment, has rapidly gained momentum since obtaining first US marketing approval in 2017. The CAR-T cell space is becoming increasingly crowded, with numerous companies competing for share of a market that is anticipated to reach four billion dollars by 2027. This crowded and rapidly evolving field necessitates effective patent strategy to protect commercial developments, manage patent timelines, and navigate the competitive patent landscape.
We discuss these strategic considerations in this three-part series to provide a blueprint for establishing an investible patent strategy, focused on protecting CAR-T innovation in US and Australian markets.
The CAR-T cell breakthrough
Chimeric antigen receptor (CAR) T-cell therapies have altered the treatment paradigm for blood cancer. It took almost a quarter of a century for first generation CAR molecules to progress to the first approved CAR-T cell use in 20171. CAR-T cell development and approval rates have since accelerated rapidly. Six CAR-T cell products have been approved by the US Food and Drug Administration (four of which are also approved in Australia) and over 1,000 clinical trials are underway globally2 (Figure 1).
CAR-T cell therapy uses immune cells called T cells which are genetically modified to carry fusion proteins on their surface called Chimeric Antigen Receptors. The receptors are “chimeric,” as they combine both antigen-binding and T cell activating functions into a single receptor. The resulting CAR-T cell specifically targets and directs killing of cancer cells (technology reviewed here3).
The commercial significance of CAR-T cell therapy cannot be overstated, with a global market of almost four billion US dollars anticipated by 20274. Breakthrough efficacy in solid tumour would warrant exponential additional growth in current estimates.
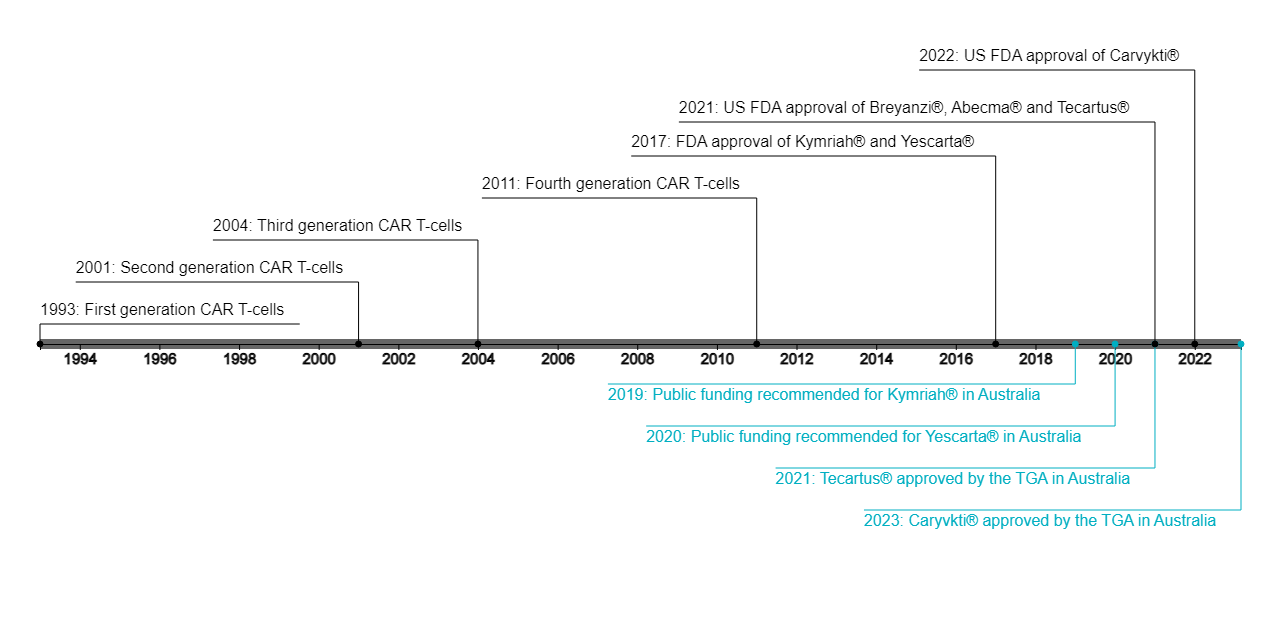
Series overview
In this article series, we discuss the following primary patent strategy considerations for companies focused on the US and/or Australian markets:
- Protecting your competitive advantage: Allowing companies to communicate why their product advantages(s) will change current clinical practice and, how these advantages can be protected with patents to establish market exclusivity.
- Managing the tension between patent term and product approval timelines by protecting new developments: Monitoring R&D outputs as products develop to ensure opportunities for patent protection are identified and, if commercially relevant, pursued.
- Understanding the patent landscape: Undertaking the level of searching required to keep up to date with the patent landscape and its impact on prospects for Freedom to Operate (FTO).
Part 1: Protecting your competitive advantage
Currently, approved CAR-T cell products have achieved unprecedented therapeutic results. To gain traction in a crowded market, companies (and investors) will need to focus on identifying new product characteristics that can change current clinical practice and secure reimbursement from commercial insurers. Patent strategy should align with key product characteristics to maximize prospects for establishing market exclusivity.
In April 2023, Evohealth published a white paper6 outlining how advances in CAR-T cell research will benefit patients. These advances and others, which are summarized below, offer tremendous up-side for patients, potential points of differentiation from competing products and, with appropriate patent strategy, an opportunity to establish some market exclusivity in various countries including the US and Australia7.
The key focus areas are discussed below, including new compositions, methods of manufacture and methods of treatment. Effective patent strategy in the CAR-T cell space leverages developments in one or more of these areas to position products for market exclusivity. As discussed further in part 2 of this series, companies must continually monitor developments in at least these areas as products evolve to ensure opportunities for patent protection are identified and, if commercially relevant, pursued.
Compositions of matter
“Composition of matter” claims are often viewed as the most valuable patent claims by investors in view of their potential breadth of coverage. Examples of new compositions that are emerging in the CAR-T cell space include:
- Composition of the CAR such as including additional costimulatory domains.
- Structural components of the CAR such as safety switches and immune-checkpoint modulation (e.g., armored CAR-T cells)8.
- Altered T-cell function such as T cells redirected for universal cytokine-mediated killing (TRUCKs)9.
- Allogeneic (or “off-the-shelf”) CAR-T cell therapies10.
- Natural Killer (NK) cells engineered to express a CAR11.
- Gene edited immune cells expressing a CAR12 (e.g., gene edited CAR-T cells).
- Dual targeting CARs and associated immune cells13.
Innovative and personalised CAR-T cell manufacturing methods
What can we learn from antibodies and, more recently, mRNA products? Manufacturing capability is critical. The autologous nature of currently approved CAR-T cell products necessitates use of complex manufacturing methods that are difficult to scale. As a result, CAR-T manufacturing capability is currently fragmented with many companies and research hospitals developing their own methods. While large pharma has invested heavily in manufacturing, there are few centers globally that can support commercial scale CAR-T cell production. Accordingly, there remains an enormous opportunity to leverage improvements in manufacturing methods and automation to gain traction in a crowded market.
Patents covering improved manufacturing methods may be incredibly valuable in the CAR-T space, particularly where improvements underpin scalable manufacturing methods that can reduce “vein-to-vein time” (i.e., time required to obtain T-cells, “weaponize” them, and reintroduce CAR-T cells). Reducing vein-to-vein time is critical to ensure patients can be treated as soon as possible and before they succumb to their disease. Australian companies such as Carina Biotech14 and Cell Therapies Pty. Ltd. have made substantial progress on this front. Examples of potentially patentable manufacturing methods include:
- Production of allogeneic (“off the shelf”) CAR-T cells15;
- Gene editing based methods of CAR-T cell production16;
- Methods of genetic modification which incorporate non-viral delivery systems;
- Next day manufacturing methods17;
- Devices/culture systems which implement the above may also be patentable as well as systems with new features such as integrated quality control measures (e.g., ability to track cell viability, cell number, cell identity, purity and/or potency).
Method of treatment patents (MoT)
Well drafted method of treatment (MoT) patents are useful tools for biotech companies. Importantly, MoT patents can protect approved therapies as well as new discoveries made after an initial approval, like dosing regimens and treatment of new patient populations. The value of MoT patents is evidenced by the commercial success of re-purposed drugs. Although re-purposed drugs are typically only patentable using MoT claims rather than composition claims, some of the world’s most commercially successful medical products were discovered through repurposing efforts. Think Viagra®18 and Retuxan® (Retuxumab)19.
Examples of MoT patents that may offer significant value in the CAR-T space follow:
- Methods which offer a broader scope of conditions that can be treated (e.g., new clinical indications such as solid tumours and applications beyond oncology)20;
- Methods which provide more durable treatment response (e.g., dual-targeted CAR-T cells)21;
- Methods which reduce morbidity (e.g., reduced toxicity and/or side-effects)22.
Patent strategy summary
Patent strategy should be aligned with product advantage(s). Effective patent strategy aims to position company leaders to communicate how their products will change current clinical practice and their plans for establishing market exclusivity. While “composition of matter” patent claims can be incredibly valuable, patent claims covering methods of manufacturing and/or methods of treatment can also add significant upside to CAR-T patent portfolios, and should not be overlooked.
Collaborate with our IP strategy team
Are you working in the CAR-T cell or broader personalised cell therapy space? Our IP strategy team is here to collaborate with you. Contact us today to steer your CAR-T breakthrough with an effective patent approach.
Footnotes
Eshhar et al. (1993) PNAS., 90:720-724; Novartis Pharmaceuticals Corporation. KYMRIAH (tisagenlecleucel) Suspension for Intravenous Infusion: Prescribing Information. Accessed August 16, 2023; Kite Pharma, Inc. YESCARTA (Axicabtagene Ciloleucel) Suspension for Intravenous Infusion: Prescribing Information. Accessed August 16, 2023.
Wang et al. (2023) Cancers (Basel)., 15:1003
June et al. (2018) Science., 359:1361-1365
Teoh and Ching (2021) Blood Cancer J., 11:84
Evohealth. 2023. CAR T-Cell Therapy: Ready, Willing and Able? Evohealth, Canberra
Examples discuss claims in terms of patent eligibility. Claims will also need to satisfy additional patentability criteria such as novelty and inventive step to progress to grant in the United States of America and Australia.
Tomasik et al., (2022) Front. Immunol., 13:1-13
Ibid
Castelli et al., (2022) Cell Research., 32:1036-1037
Xie et al., (2020) Lancet., 59:102975
Alzubi et al. (2020) Molecular Therapy: Methods and Clinical Development., 20:379-388
Sterner and Sterner (2021) Blood Cancer Journal., 11:69
Carina Biotech’s proprietary CAR-T manufacturing process: Accessed August 16, 2023
Qasim et al. (2017) Sci Transl Med., 25:374
Alzubi et al. (2020) Molecular Therapy: Methods and Clinical Development., 20:379-388
Yang et al. (2022) Blood Cancer J., 12:104; Ghassemi et al. (2022) Nature Biomedical Engineering., 6:118-128
Over the past two decades, Viagra® has consistently generated annual sales to the tune of $1.8bn. The active ingredient in Viagra® was originally developed to treat angina but was repurposed to treat erectile dysfunction.
Rituxan (Retuxumab) hit $6.54 billion in sales in 2019, making it the second-best selling drug in 2019. Retuxumab was repurposed to treat rheumatoid arthritis.
Zmievskaya et al. (2021) Biomedicines., 9:59
Sterner and Sterner (2021) Blood Cancer Journal., 11:69
Ibid

