CAR-T cell therapy: Manage your patent term to create an investible patent strategy
Effective patent strategy manages the tension between the challenging commercial realities of long development times and the finite nature of patent term. This second installment in our investible patent strategy series discusses how new product developments can be leveraged to ensure that sufficient patent coverage is available for investors and commercial partners after product approval.
Part 2: Managing patent term by protecting new developments
Every CAR-T company can trace their origins to a light-bulb-moment, an idea. Funds are raised, R&D projects begin, and patents are filed as founders start down the long road towards revenue generation. Attention inevitably turns to raising further capital or partnering. It is common for new investors and partners to enquire about estimated product approval timeline and, how much patent exclusivity is available if the estimated timeline is correct. While not all founders (or investors) will stay on board for the entire journey, the finite nature of patent term requires careful management to ensure that sufficient patent term remains for the company and/or their commercial partner after product approval. This article discusses strategies for managing the tension between patent term and product development timelines by pursuing patent protection for new developments.
The tension between patent term and development timelines
Patent protection is granted for a limited period, generally 20 years from the filing date of the application1. However, recent analysis of clinical development times (i.e., number of years between first in human trials and approval) for US FDA approved drugs over the last decade notes a stable median development time of 8.3 years, with many products taking upwards of 15 to 20 years2. Add in pre-clinical work and the development timeline is extended further. Effective patent strategy manages the tension between the challenging commercial realities of long development times and limited patent term.
Aligning patent strategy with product development
Certain jurisdictions offer patent term extension (PTE) provisions which can extend a patent covering an approved product by up to 5 years (Reviewed here3). However, PTE is not available in all instances and, is subject to certain criteria which dictates the availability and length of extension. Accordingly, secondary patents (i.e., “applications covering new developments in technology filed after an initial application”) play a significant role for CAR-T cell companies.
The following stages of CAR-T cell product development can be monitored to ensure opportunities for secondary patent protection are identified and, if commercially relevant, pursued.
Pre-clinical Stage
Pre-clinical stages of CAR-T cell development are reviewed here4. Some recent developments include:
- Potency, safety, and durability: Because a CAR transgene is permanently integrated into the T cell genome, pharmacokinetics and pharmacology studies are generally used to assess cell infusion, trafficking, proliferation, persistence, and apoptosis5. These data, which may also arise during clinical development, can support new filings to compositions with increased potency/safety/durability and methods of treatment based on the same.
- New targets: Target identification/validation work may underpin new compositions directed to new or additional targets.
Manufacturing Stage
The personalised nature of CAR-T therapy often requires companies to develop bespoke manufacturing capabilities around individual patients or patient populations. Whilst changes to process steps may be considered trivial or routine, it is worth considering their overall impact on the manufacturing process as new and improved processes may result in commercially important developments. Examples include:
- Methods which reduce “vein-to-vein time” (i.e., time required to obtain T-cells, “weaponise” them, and reintroduce CAR-T cells).
- Alternative methods of modifying cell gene expression (e.g., non-viral delivery)
- Methods which improve product potency or yield.
Clinical Stage
Clinical trial results can also be fertile ground for new inventions. Examples include:
- New indications and patient populations: Clinical trial data may underpin use in new indications (e.g., use in different cancers) or patient populations (e.g., use in certain patients).
- Dosing regimens: Protection may be sought if a product provides improved patient outcomes or surprising or unexpected effects at a particular dose (e.g., a surprisingly low dose) or dosing interval.
- Improved safety: Methods which reduce patient morbidity (e.g., reduced toxicity and/or side-effects).
- Combination therapy: Incorporating an additional product into a composition or treatment regimen may offer another level of protection, in particular when the efficacy of the product combination exceeds that anticipated based on results achieved with each product alone (e.g., synergy).
Advantages of effective patent term management
The benefits of managing patent term by protecting new developments are highlighted in Figure 2. The depicted scenario assumes a predicted date of initial marketing authorisation in 2038. Subject to any applicable extensions, an original patent covering a CAR-T cell composition, filed in 2022, will expire around 2042. Filing subsequent applications covering a new use of the composition (e.g., a new method of treating solid tumours) in 2026 and a new method of manufacturing the CAR-T cell in 2028 may provide useful patent protection through 2046 and 2048 respectively (i.e., a 6-year extension in patent coverage over the original filing). With this hypothetical strategy in play, a CAR-T cell company can pitch to potential investors/partners based on projected patent coverage for 10 years post-approval (i.e., 4 years based on its initial filing and a further 6 years based on its secondary patents directed to methods of treatment and manufacturing). Considering the anticipated growth in the global CAR-T cell market, the additional patent term obtained from a well-designed patent strategy can significantly increase the value of the underlying CAR-T cell product.
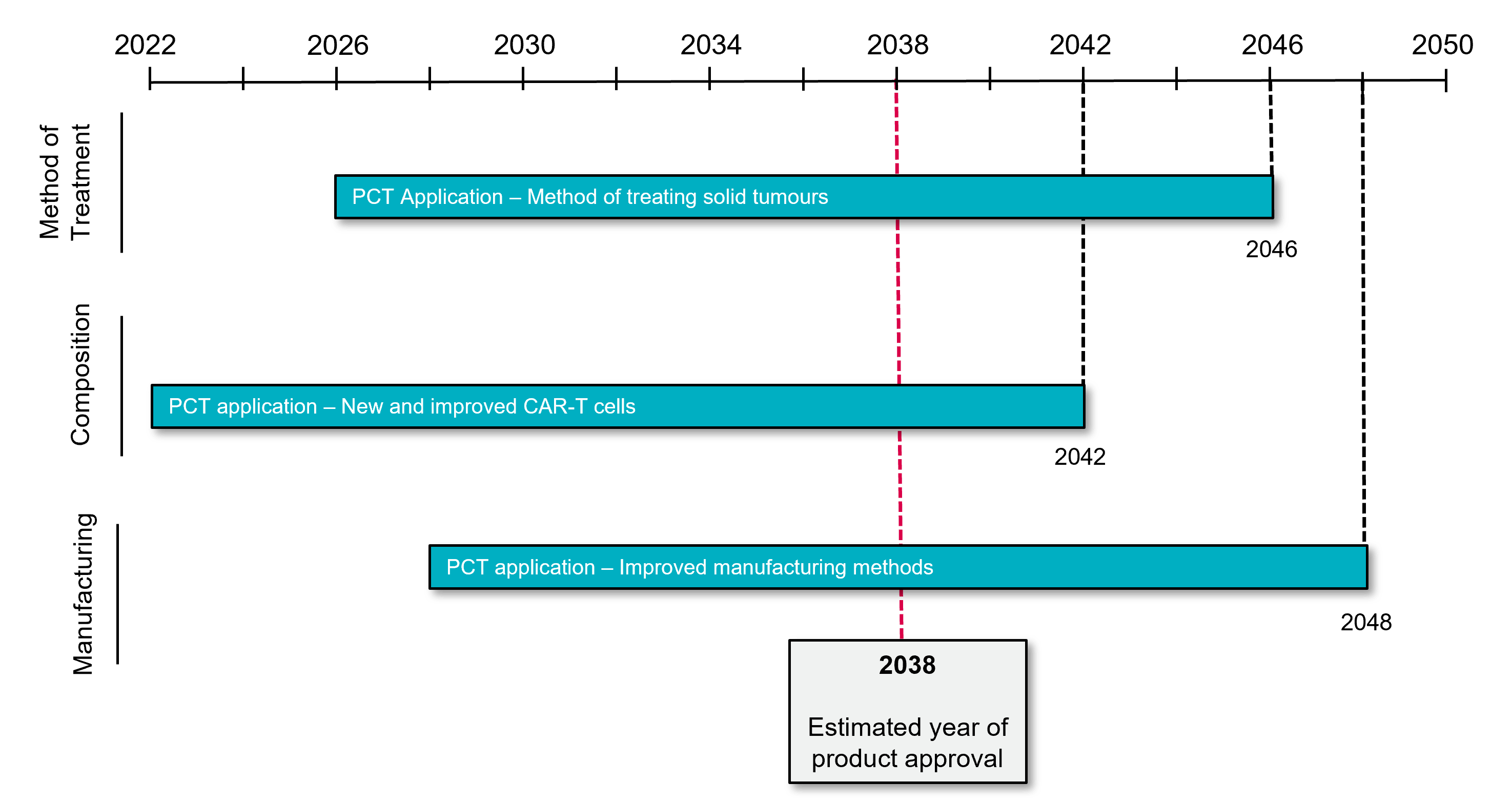
Patent strategy summary
Commercial CAR-T cell development is a long road. The finite term of patents requires careful management to ensure that sufficient patent coverage is available after product approval. The tension between patent term and product approval timelines can be managed by obtaining patent protection for new developments identified thorough careful monitoring of pre-clinical, manufacturing, and clinical development programs.
Collaborate with our IP strategy team
Are you working in the CAR-T cell or broader personalised cell therapy space? Our IP strategy team is here to collaborate with you. Contact us today to steer your CAR-T breakthrough with an effective patent approach.
Footnotes
Certain countries such as the US and Australia have legislated provisions for extending patent term up to 5 years following marketing approval.
Brown et al. (2022) Nature Reviews: Drug Discovery., 21:793-794.
Article: You can’t spell patent without PTE (Accessed August 17, 2023)
Ibid.

